To function properly, our brain needs a tightly controlled environment. Toxic components circulating in the blood are detrimental to this homeostasis and need to be withheld. The blood-brain barrier, a network of specialized blood vessels separating the brain from the rest of the body, is a key determinant of this neuroprotection.
Blood-brain barrier (BBB) dysfunction is a hallmark of numerous severe brain disorders, generating inflammation and major dysfunctions. However, no treatment options are currently available to target this system. Research on the topic is emerging and has the potential to provide significant therapeutic benefits to patients.

The importance of Gpr124/Reck as new targets for BBB repair
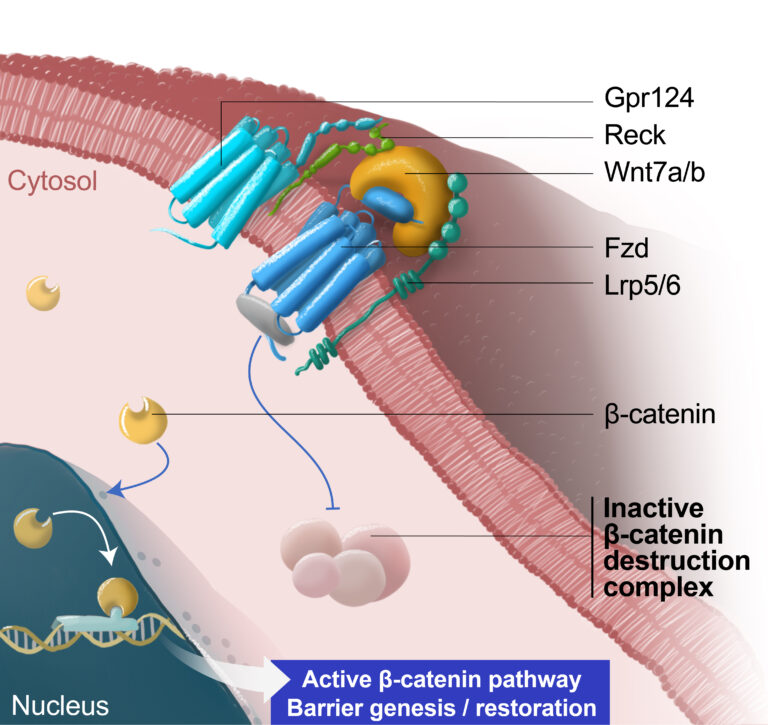
Neuvasq’s scientific research is based on the groundbreaking discovery that the endothelial Wnt/β-catenin signaling pathway plays a crucial role in regulating the formation of blood vessels in the brain, including those of the BBB.
Recent studies have advanced our understanding of this pathway to the point where it appears possible to develop safe and effective therapeutic interventions. In particular, researchers have identified key components of this Wnt/β-catenin pathway, called Gpr124 and Reck, representing a prime therapeutic target for BBB therapy.

The in vivo validation of Gpr124/Reck activators
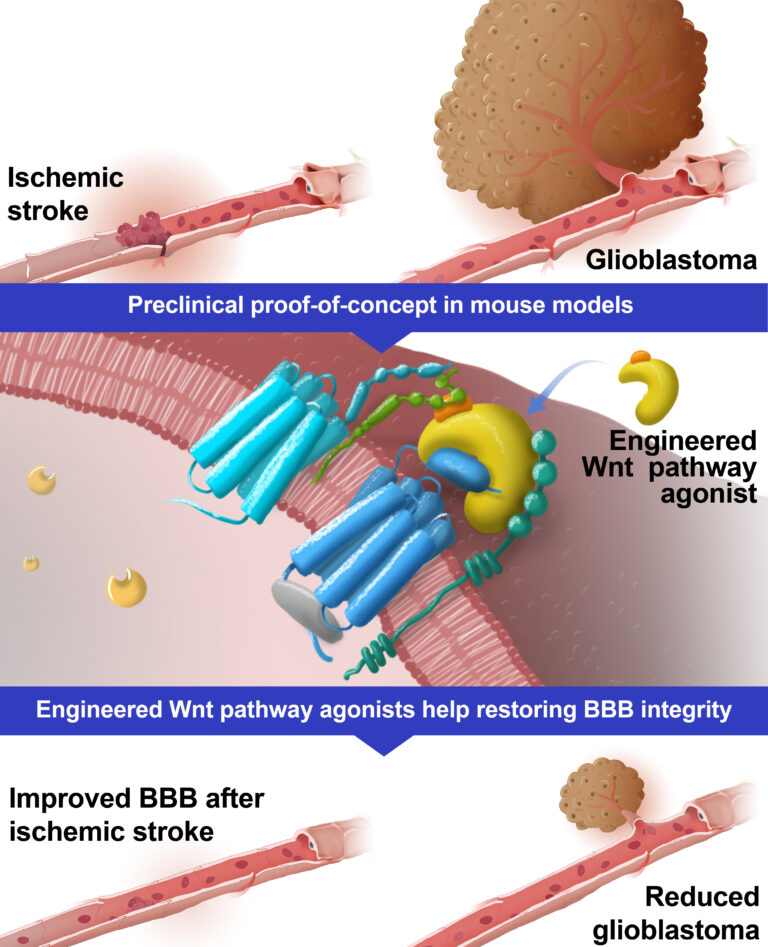
The team led by Benoit Vanhollebeke at Université libre de Bruxelles (ULB) recently developed a novel class of highly specific and fully active Gpr124/Reck agonists, inducing safe activation of the Gpr124/Reck complex at the BBB. In preclinical models of stroke and brain tumors, these activators showed good tolerance and clear signs of BBB normalization, leading to positive therapeutic effects.
This strong scientific evidence lead to the foundation of Neuvasq, where insights are translated into pioneering therapies that address different neurovascular, neurogenerative, and neuro-oncological disorders.

Scientific references
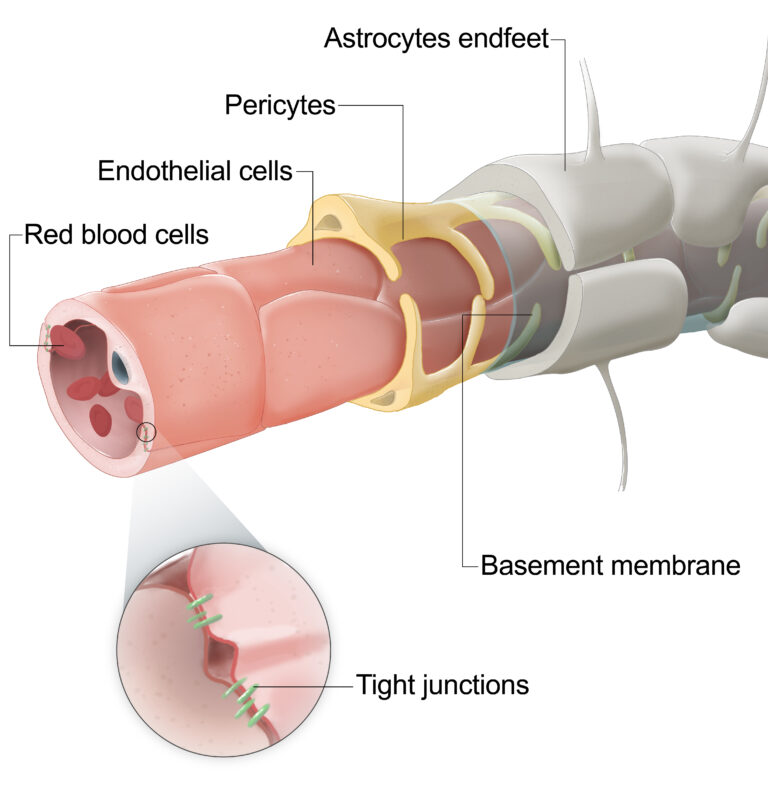
The central nervous system (CNS) operates as a tightly controlled environment, in which several cell types help regulate the exchange of substances between the blood and the CNS. These cell types maintain the integrity of CNS barriers, enabling normal neurological function.
Examples of CNS barriers include the blood-brain barrier (BBB) and the blood-retinal barrier (BRB).
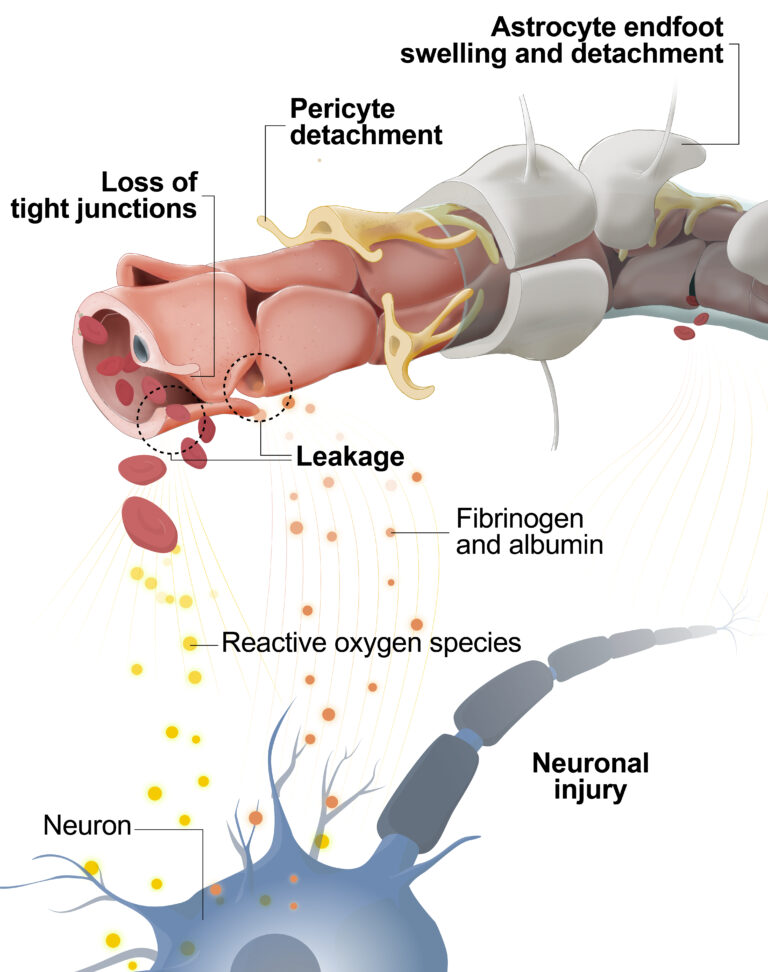
Dysfunction of CNS barriers is a hallmark of numerous severe neurological diseases associated with inflammation and neurodegeneration. BBB breakdown has been implicated in Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, and epilepsy, among other conditions. BRB impairment is associated with vascular retinopathies such as diabetic macular edema (DME) and wet age-related macular degeneration (wAMD).
Most current treatments for the above-listed diseases are designed to ameliorate inflammation, neurodegeneration, or abnormal angiogenesis rather than restore the BBB.
No treatment options are currently available to safeguard or rebuild CNS barriers. Discovery and development of novel approaches targeting the BBB or the BRB has the potential to deliver significant therapeutic benefits to patients.
A scientific team from the Université libre de Bruxelles (ULB), led by Benoit Vanhollebeke (founder of Neuvasq), leveraged this discovery to develop a novel class of highly specific Wnt pathway agonists that selectively activate the Gpr124/Reck complex at the BBB.
The ULB team, in collaboration with other international universities, showed that AAV-based expression of these novel Wnt pathway agonists was well tolerated and led to positive therapeutic effects in mouse models of stroke and brain tumors.
Following its foundation, Neuvasq was granted an exclusive, worldwide license to research, develop and commercialize Wnt pathway agonists under ULB patents rights and associated know-how.
Neuvasq is developing novel Wnt pathway agonists to selectively activate the Wnt/β-catenin pathway at the BBB or BRB, promising to restore neurovascular function for patients with severe neurological conditions.
Leveraging these scientific discoveries, Neuvasq has initiated research programs to deliver novel small molecules and biologics that can selectively target and support the proper functioning of the BBB.